Understanding Risk Factors
The concept of risk factors is based on statistical probabilities. Clinical studies have demonstrated associations between certain biochemical, physiological, and environmental factors and coronary heart disease. By definition, the presence of one or more of these factors increases the risk of clinical manifestations of atherosclerosis.
Risk factors for coronary artery disease include:
-
-
- Diabetes. Diabetes is the most important risk factor for cardiovascular disease. According to the American Diabetes Association, ‘2 out of 3 diabetics will die of heart disease and stroke’. Coronary artery disease develops more frequently and at an earlier age in those with diabetes than in other individuals. Chronic hyperglycemia results in abnormal fat metabolism leading to deposition of lipids in the vascular walls.
-
-
-
- Family history. Coronary artery disease often occurs in several members of the same family. Studies have shown a familial history of heart disease in over 50% of patients with atherosclerosis, particularly due to the inheritance of hypertension or hyperlipidemia.
-
-
-
- Age and gender. The incidence of heart disease increases with advancing age in both men and women. Men have a greater incidence of heart disease when compared to premenopausal women of similar age. Estrogen is thought to be a protective influence in premenopausal women. After menopause, however, the incidence of heart disease begins to increase in women. The incidence in men and women is roughly equal when they reach their 70s.
-
-
-
- Elevated serum cholesterol. Sustained increases in serum cholesterol levels, particularly LDL-cholesterol, are a risk factor for development of progressively severe atherosclerotic lesions and the classic signs and symptoms of coronary artery disease. Low levels of HDL-cholesterol (good cholesterol) also are a risk for coronary artery disease.
-
-
-
- Hypertension. As blood pressure rises, whether systolic or diastolic, the risk of developing ischemic heart disease also increases. Hypertension may contribute to its development in two ways: by predisposing arteries to atherosclerosis and by increasing the workload on the left ventricle.
-
-
-
- Cigarette smoking. The evidence that cigarette smoking greatly increases the incidence of coronary artery disease makes smoking perhaps the most important preventable coronary artery disease risk factor. Smokers have more than twice the risk of developing coronary artery disease than nonsmokers. In addition, cigarette smoking is the strongest known risk factor for sudden cardiac death. Sudden cardiac death is two to four times more likely in smokers than in nonsmokers.
-
-
-
- Obesity. Cholesterol and fats become deposited beneath the intima at many sites in the arteries of obese patients. These fatty deposits eventually become invaded by fibrous tissue and frequently become calcified. The net result is the development of both atherosclerotic plaques and very stiff arterial walls that can be neither constricted nor dilated. The internal diameter of the artery is almost always greatly narrowed, thereby decreasing the rate of blood flow.
-
-
-
- Personality and lifestyle. Sedentary occupations and lifestyles contribute to the development of atherosclerotic heart disease. Many cardiologists feel that while regular physical exercise may not prevent the occurrence of a myocardial infarction, it might increase the individual’s chances of recovery because regular exercise leads to the development of collateral circulation. Although it is a commonly held belief that there is a relationship between a Type A behavior pattern and an increased incidence of ischemic heart disease. An individual who has this type of personality is described as aggressive, highly competitive, and acutely aware of the passage of time.
-
Ischemia
While ischemia and infarction may, and often do, occur simultaneously, the scheme is somewhat oversimplified. Nevertheless, this spectrum is useful for understanding the clinical picture of ischemic heart disease and the signs and symptoms that bring a patient to the cardiologist.
Ischemia occurs when the coronary blood flow is diminished due to a narrowing of the arterial lumen by an atherosclerotic plaque or to occlusion by an embolus. The result is diminution of the blood supply to an area of the ventricular myocardium. In general, the symptomatology reflects the extent of coronary artery disease, the location of the disease, and the degree of occlusion.
As the size of the atherosclerotic occlusion increases, the degree of myocardial perfusion (blood flow) decreases and the patient experiences more severe symptoms. These symptoms are in the form of chest discomfort, better known as angina pectoris. Blood flow through the coronary arteries normally increases as the work of the myocardium, and its need for oxygen, increases. In a patient with severe coronary artery disease, even low levels of exertion will lead to myocardial oxygen debt because the limited amount of blood flow cannot meet the demand. Therefore, anginal pain will occur earlier and much more frequently.
The main determinants of myocardial oxygen demand in the ischemic heart include:
• Contractile state of the myocardium
• Heart rate
• Tension of the myocardial wall
In addition, the risk of developing a myocardial infarction increases with the severity, extent, and duration of myocardial ischemia. Infarction is the end point of moderate to severe ischemia over a prolonged period of time.
Angina Pectoris
Normally you cannot feel your heart, but ischemic cardiac muscle does produce pain. It is believed that ischemia causes the muscle to release lactic acid and other pain-promoting substances that are not cleared very rapidly due to diminished blood flow in the ischemic area. The concentrations of these substances build up and stimulate nerve endings in the cardiac muscle. Pain impulses are conducted through the sympathetic afferent nerve fibers to the central nervous system. As a symptom, angina is the intense pain itself, located beneath the breastbone and described as oppressive, vicelike, or squeezing. That pain may be misinterpreted by the patient or even by the physician as indigestion, muscle pain, arthritis, or esophageal spasm. As a syndrome, angina is ischemic heart disease manifested primarily by this particular form of chest pain. In most persons who develop progressive constriction of the coronary arteries, cardiac pain (angina pectoris) begins to develop when the workload on the heart exceeds what is sustainable by coronary blood flow to the heart muscle.
When an individual with coronary artery disease is at rest, delivery of blood to the heart is usually adequate. However, when cardiac work is increased, the stiffened, diseased coronary arteries are unable to respond with an increase in diameter or with an augmented delivery of oxygenated blood to the heart muscle. In effect, demand is greater than available supply, and ventricular muscle becomes deprived of the oxygen necessary to maintain the work of pumping.
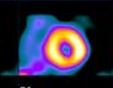
There are four major features of the anginal process: Site, Character, Duration, Exertion The Anginal Pattern (Shaded Areas) Representative of Myocardial Ischemia
Site The Levine sign, in which a clenched fist is held over the sternum, is the classic gesture that is indicative of myocardial ischemia. This discomfort is usually felt beneath the upper portion of the sternum, and it often radiates down the arms (usually the left arm) and up the neck, the shoulder, the jaw, and sometimes the back. The reason for this distribution pattern is that, during embryonic life, the heart originates in the neck, as do the arms. Therefore both of these structures receive pain nerve fibers from the same spinal cord segment as the heart.
Character The discomfort is often described as a squeezing, crushing, strangling, tightness, pressure, heaviness, or a vague ‘funny feeling’. It is usually moderate to severe and is not described as a pain by many patients. The sensation noted in the neck is frequently described as choking; in the jaw, as a toothache; and in the arms, as a numbness or tingling sensation. Exertion Angina is usually brought on by some form of exertion, often walking uphill. However, there are other provoking factors such as emotional stress, cold weather, eating a heavy meal, and public speaking. The discomfort begins during the stress as opposed to after the activity.
Duration
The discomfort usually lasts 2 to 10 minutes but can last as long as 20 to 30 minutes, especially when associated with emotional stress. Typically, the symptoms subside with rest within 1 to 15 minutes. Nitroglycerin relieves the anginal process within 5 to 10 minutes.
Even in its mildest forms, angina is a warning, a natural inducement for the patient to rest and prevent a recurrence of symptoms or even irreversible damage. The need for such a warning is clear: Only 50% of patients are alive 7 years after their first anginal episode.
Infarction
• Death of the tissue (infarction) occurs in the area immediately affected.
• The infarcted area is surrounded by an area of injured muscle.
• This injured area is usually bordered by a hypoxic (ischemic) area.
 |
 |
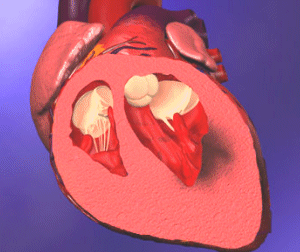
|
| Effects of Myocardial Infarction, Injury, and Ischemia of the Left Ventricle | The Infarction Process Involving the Transmural and Subendocardial Portions of the Myocardium |
[ARTWORK ATTRIBUTION: The Heart of Nuclear Cardiology: An Interactive Primer, Version 1.0, 2002, Bristol-Myers Squibb Imaging, Inc. Review of Nuclear Medicine Technology.]
The overall process is irreversible tissue death called myocardial infarction. Infarction occurs only in the center of the infarcted area. There are an increasing number of normal or near-normal cells toward the periphery, and it is this borderline zone that is most susceptible to pharmacologic and physiologic intervention to maintain myocardial function and prevent necrosis.
When the entire thickness of myocardial muscle is involved in this process, the result is a transmural infarction. Infarcts that affect only a part of the thickness of the wall are called subendocardial infarcts.
Transmural myocardial infarctions are usually associated with atherosclerosis involving a major coronary artery. They are also subclassified, depending on location, into anterior, inferior, lateral, septal, and posterior wall infarctions. The location is determined on the basis of EKG patterns and myocardial perfusion imaging.
Subendocardial infarction involves small areas, particularly in the subendocardial wall of the left ventricle, the ventricular septum, and the papillary muscles that regulate the opening and closing of heart valves.
An acute heart attack is characterized by the sudden onset of pain similar to angina pectoris, except that it is usually more severe, persists longer, and is generally not relieved by nitroglycerin or rest. Profuse sweating, belching, nausea, shortness of breath, and a pale, ashen coloration may accompany the attack.
Soon after the onset of the infarction, small amounts of collateral flow seep into the infarcted area, and this, combined with progressive dilation of the local blood vessels, causes the area to become overfilled with stagnant blood. Simultaneously, the muscle fibers utilize the remaining oxygen in the blood, causing the hemoglobin to become totally reduced and very dark blue in color. Therefore, the infarcted area takes on a dark bluish color, and the blood vessels in the area appear to be engorged, despite the lack of blood flow. In later stages, the vessels become highly permeable and leak fluid. The tissue becomes edematous (fluid filled) and the cardiac muscle cells begin to swell because of diminished cellular metabolism. Finally, many of the cells die.
Irreversible structural changes in cell metabolism occur after 20 minutes of ischemia. However, it is known the process of myocardial infarction is a dynamic process that may progress over at least 24 hours. According to recent studies, the size of an ischemic zone may continue to increase for up to 18 hours. The region of central necrosis extends out from the central area.
These data, plus the lack of a clear demarcation between infarcted and normal tissue, have promoted the concept of an ischemic myocardium surrounding the central necrotic area of infarction. Presumably the quantity of oxygen delivered to this area, plus the oxygen demands of the ischemic area based on myocardial work requirements, determine the eventual outcome of the twilight area. The assessment of this ischemic region to direct myocardium-preserving efforts is one of the principal rationales for myocardial perfusion imaging in the acute myocardial infarction setting.
Complications Following Acute Myocardial Infarction
The size and location of the area involved determine the effects and complications of myocardial infarction. Pain and at least a mild, temporary decrease in the pumping power of the heart occur in most cases. More serious effects also occur in many cases, sometimes leading to death despite every effort to save the patient.
Arrhythmias Over 75% of patients who have an acute myocardial infarction develop arrhythmias sometime during the first 72 hours of the infarction. As many as 50% of patients with an infarction develop a potentially lethal ventricular arrhythmia at the onset of infarction, and this arrhythmia can precipitate sudden death within minutes of its onset. The seriousness of any arrhythmia depends not only on its type but also on the degree of myocardial dysfunction. A number of clinical factors are associated with increased potential for developing an arrhythmia. These include conduction abnormalities, systemic hypoxemia, cardiomegaly, left ventricular failure, acidosis, increased sympathetic nervous system stimulation, and hypokalemia. Even relatively small infarcts may produce severe, life-threatening arrhythmias, as localized hypoxia and electrolyte disturbances cause irregularities of impulse formation or impulse conduction due to damage of the conduction system.
Pulmonary Edema In the normal heart, the output of the right and left ventricles is equal. When the pumping action of the left ventricle is weakened by myocardial infarction, blood may accumulate in the pulmonary circulation, causing pulmonary congestion. Expressed more fully, incomplete emptying of the heart causes an increase in venous blood volume at the expense of the arterial blood volume. Decreased arterial volume and pressure stimulates secretion of aldosterone from the adrenal glands causing retention of sodium and water and an increase in total blood volume. Excessive venous blood volume results in edema, the shift of fluid from the capillaries to the tissues due to increased hydrostatic pressure.
Cardiogenic Shock Left ventricular function may be severely altered due to loss of ventricular contractility, arrhythmias, conduction defects, or ventricular aneurysm. Any of these conditions may lead to an extensive decrease in cardiac output, with resulting inadequate tissue perfusion throughout the body. Insufficient blood flow to the coronary arteries may cause further myocardial injury and inadequacy of pumping, thus initiating a vicious cycle leading to irreversible shock and death.
Ventricular Aneurysm The infarcted area of the ventricle is usually akinetic—that is, it fails to contract synchronously with the heart muscle tissue. At times, this relatively thin scar tissue may bulge out paradoxically whenever the remaining healthy muscle tissue contracts, thus gradually forming an aneurysm.
Mitral Insufficiency Infarction and/ or rupture of a papillary muscle or the chordae may cause sudden incompetence of the mitral valve. Rupture of a papillary muscle usually causes sudden pulmonary edema and cardiogenic shock, and may be fatal.
Ventricular Septal Defect A myocardial infarction in the septum between the ventricles may weaken the septum so much that it ruptures, creating a sudden communication between the right and left ventricles.
Cardiac Rupture Muscular necrosis resulting from a large transmural infarction may leave the ventricular wall so weakened that it ruptures. Sometimes the rupture is small and contained in a small sac, forming a pseudoaneurysm. A large rupture is often fatal.
BIOCHEMICAL MARKERS OF MYOCARDIAL INFARCTION:
Laboratory Evaluation
Several laboratory tests provide information on the cardiopulmonary status, the presence of concomitant disease or complications of myocardial infarction (MI), and the patient’s response to therapy. The following descriptions pertain only to those biochemical markers that provide diagnostic information on patients with suspected heart disease. Once a diagnosis is made and the patient is placed in the appropriate critical care area, other routine blood tests are performed to monitor the patient’s status.
Biochemical Markers
Acute MI causes enzymes and proteins to be released from the dying heart cells into the systemic circulation. These biochemical markers can be measured to determine the evolution and extent of myocardial necrosis. Serial blood tests are run in an emergency department setting in cases where acute ischemia may be suspected to rule out MI. If any of these cardiac enzymes are elevated, the patient may have had a very recent MI and must not have a stress test.
Total creatine kinase (CK): Typically becomes elevated within 8 hours after acute MI, peaks at 24-30 hours, returns to normal in approximately 3 days
Creatine kinase isoenzyme (CK-MB): Gold standard for diagnosis of acute MI, peaks at 9 to 30 hours, and returns to normal levels between 48 – 72 hours
Lactate dehydrogenase (LDH): Levels become elevated 8 –18 hours post-MI, peak within 3 days, return to normal in 6 – 10 days
Troponin T and I: More sensitive and specific than CK-MB; detect minor heart damage. Troponin I is highly specific for myocardial tissue.
Myoglobin: low specificity for cardiac muscle damage
BIOCHEMICAL MARKERS FOR THE
DIAGNOSIS OF ACUTE MI